Fluctuation analysis of mechanosensing-directed remodeling of the cytoskeleton
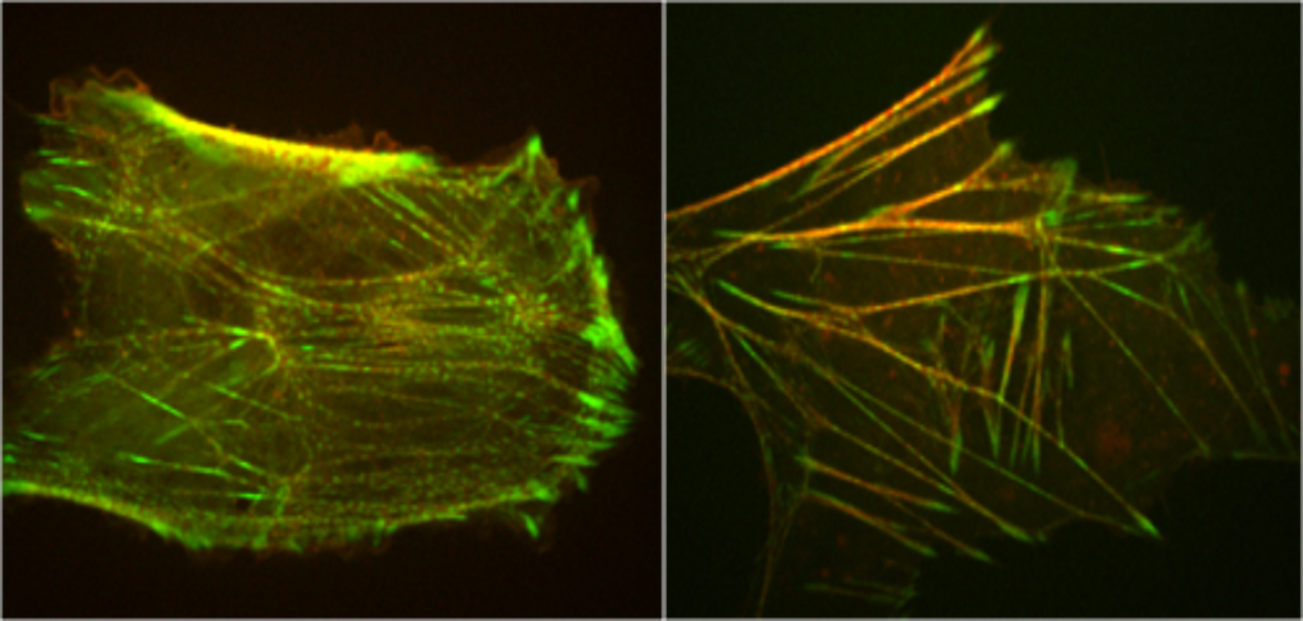
The actomyosin cytoskeleton is highly dynamic and undergoes constant remodeling. How do actomyosin cytoskeletal networks remodel themselves? Using stress fibers as a model cytoskeletal structural motif, we use quantitative analysis of experimental images and mathematical modeling to show that stress fibers use mechanosensing to detect compressive or expansive stress states, and adjust actin assembly/disassembly rates accordingly to maintain density homeostasis and avoid fracture. Thus, coordinated mechanical and biochemical signaling enables extended actomyosin assemblies to adapt dynamically to the mechanical stresses they convey and direct their own remodeling.
Our method combines statistical analysis of experimental data and mathematical modeling, and is an example of fluctuation analysis. In this approach fluctuations about steady state are measured in wild type untreated cells, and correlations between different properties are extracted to identify causal relations. This avoids the use of pharmacological treatments or genetic mutations which can radically modify the cellular system one seeks to understand.
Mechanosensing and regulation of cell wall growth during cell division
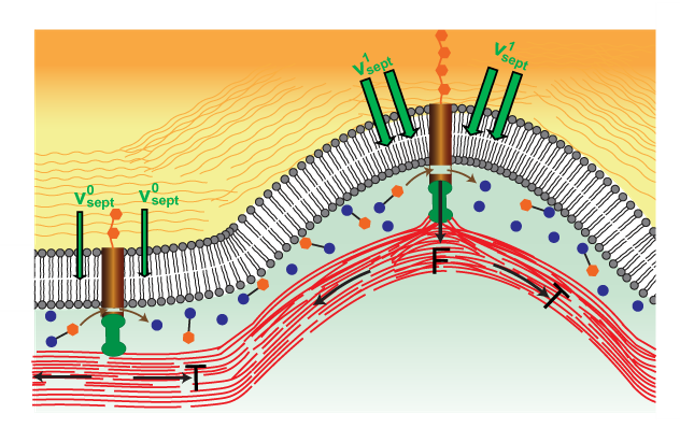
In fungi and bacteria cell division includes septation, the growth of new cell wall that ultimately encloses the two daughter cells. In fission yeast, new cell wall grows in the wake of the constricting ring. A major question is how septation is regulated to maintain an almost perfectly circular, shrinking hole. We have analyzed the mechanism of septation and its regulation in fission yeast by combining experiments on live cells, quantitative analysis of experimental images and mathematical modeling.
Our analysis provides evidence that beta glucan synthase (bgs) complexes that grow new cell wall are mechanosensitive, such that local growth rates depend on the force exerted on them by the contractile ring. An analytical mathematical model shows that local septum growth rates are modified by the ring tension in a curvature dependent fashion so that septum roughness is suppressed and circularity is maintained. Predictions of the model, which is closely related to the Edwards Wilkinson and KPZ models of stochastic interfacial growth, are in close agreement with measurements of septum roughness in live cells.
The picture emerging from this work is that, in fission yeast, the constriction rate is set by the septum-growing machinery. The role of ring tension is not to fix the constriction rate, a common view. Rather, ring tension serves (among other roles) to regulate proper septum closure so that the daughter cells are properly enclosed by cell wall after division. Thus, constriction rates cannot be used as a proxy for ring tension (a common measure, given that ring tension measurements are usually unavailable).
© O'Shaughnessy Group 2018