The actomyosin contractile ring
Cell division is essential to life as the mechanism that propagates the genome and lets organisms grow and repair. Physical division of the cell occurs at the end of the cell cycle during cytokinesis, driven by a remarkable force-generating cellular machine, the actomyosin contractile ring. We develop molecularly explicit simulations of the contractile ring, and more coarse-grained analytical descriptions guided by the lessons learned from the more detailed simulations.
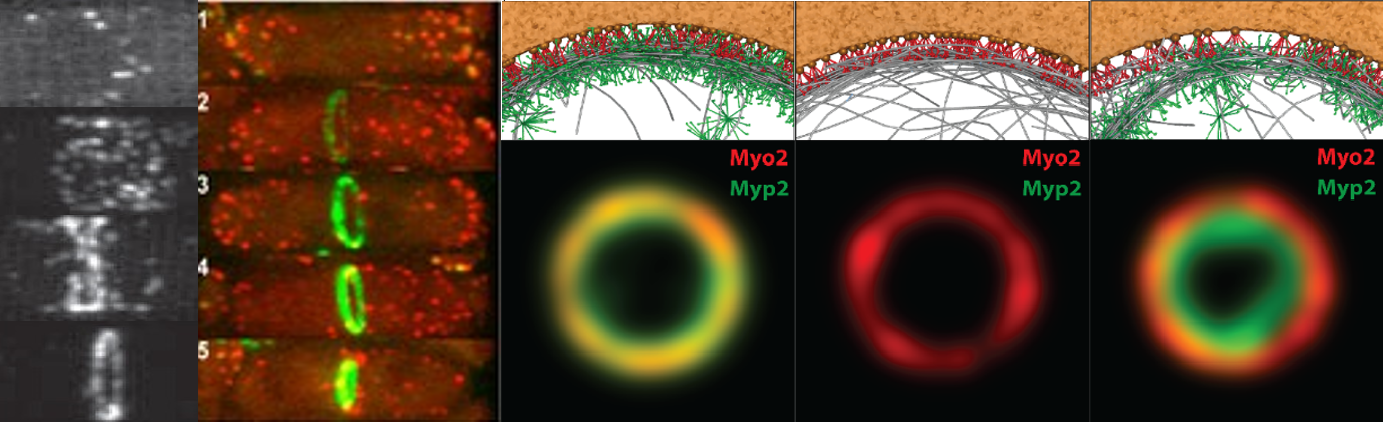
How does the contractile ring generate tension and inward force? As the ring constricts and shortens, how does it disassemble without destroying its ability to exert tension? What sets the rate of constriction? How does the organization of the ring come about, how is it maintained, and how is it related to tension production?To address these and other fundamental questions about this machine, we use computational modeling, mathematical analysis, image analysis, data analysis and experiment to model the fission yeast contractile ring. Fission yeast is presently the only organism for which realistic mathematical modeling of the ring is possible, due to the wealth of information about the components in the ring, their amounts, their biophysical and biochemical properties and, more recently, their organization from super-resolution microscopy. Lessons from fission yeast will likely carry over to other organisms, including humans, since many cytokinesis proteins are conserved.
Extraordinary dynamics of isolated contractile rings
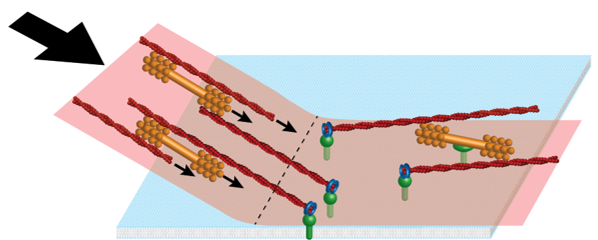
The contractile ring operates in a narrow physiological window to perform its job. Like many machines, forcing it to operate in remote regimes can expose features of the mechanism normally hidden. Fission yeast cell ghosts offer such exciting possibilities for the contractile ring. Ghosts are cells whose walls have been digested away and membranes permeabilized, releasing the cytoplasm and its contents. Should a contractile ring be present, however, it remains anchored to the plasma membrane under conditions of nearly complete isolation.
In experiments by the Balasubramanian/Mabuchi labs, isolated rings in cell ghosts became partially unanchored from the membrane and constricted much faster than normal (Mishra et al., Nat. Cell Biol., 2013). We developed a mathematical model and simulation which showed that partially anchored rings constrict in an extraordinary non-contractile mode where unanchored segments are reeled in at the load free velocity of myosin-II, reproducing experimental rates. The observed behavior provides quantitative evidence that the mechanism of tension generation in (normally anchored) contractile rings is based on a specific anchoring scheme.
© O'Shaughnessy Group 2018